This post is part of a larger deep dive
Curious about the role of the WWII atomic bombings in Raphaelesque Head Exploding? Check out Raphaelesque Head Exploding explained!
Or read the full Raphaelesque Head Exploding article!
This post is part of a larger deep dive
Curious about the role of the WWII atomic bombings in Raphaelesque Head Exploding? Check out Raphaelesque Head Exploding explained!
Or read the full Raphaelesque Head Exploding article!

Japan entered the Second World War with the objective to occupy the Western colonies of Great Britain, France, the Netherlands and the US, all situated in South east Asia. Not only was this a move to expand its empire, but also to cease key natural resources (e.g., oil, coal) that Japan desperately needed if it were to become a great world power.
In 1931, Japan invaded Manchuria, a place rich in coal and iron. The US became unease, since they suspected Japan would take advantage of the war in Europe to capture the colonies of defeated European countries.
So, US president Franklin Roosevelt decides to send his Pacific naval fleet to Pearl Harbor, Hawaii, to be stationed there for training… and keep an eye on the Japanese.
Meanwhile, Japan makes a pact with Nazi Germany and Fascist Italy, boosting Japan’s intentions to continue their territorial expansion. As Roosevelt had predicted, after the fall of France to the Nazis, Japan invades French Indochina.
As retaliation, the US suspends all exports to Japan, effectively blocking Japan’s ability to purchase oil (which had mostly come from the US). Having thousands of troops stationed in China and other occupied territories, Japan desperately needed oil to sustain the presence of those troops.

Based on the belief that the Americans would not be willing to get dragged into a lengthy war in the pacific, the Japanese military gambled that if they could destroy the US pacific fleet (now conveniently stationed in Pearl Harbor), the US would soon ask for peace, clearing the way for Japan’s expansion ambitions in the Pacific.
Japan’s surprise attack on the US naval base at Pearl Harbor, Hawaii on the 7th December 1941, sent a shockwave through the US.
Having been reluctant to enter the war prior to the Pearl Harbor attack, there was no question now. On the next day, the US declared war on Japan and officially entered WWII.
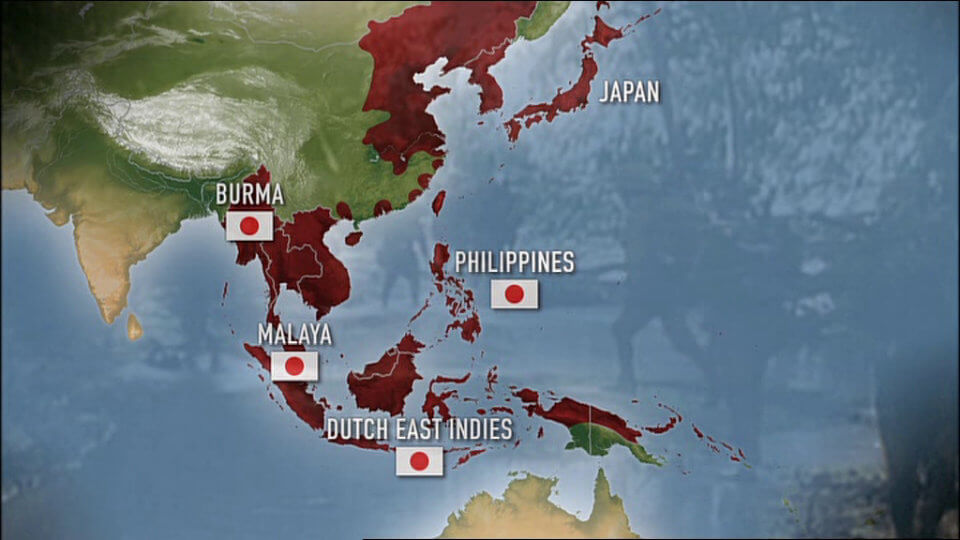
Believing to have crippled the US, Japan continued their expansion in South Asia.
In just under 6 months they had invaded and conquered Hong Kong, Burma, Thailand, Malaya, Singapore, the Philippines, the Dutch east Indies, the Solomon islands and other smaller pacific islands.
However, during those Japanese campaigns in South Asia, American codebreakers in Hawaii managed to crack Japanese encoded Japanese radio messages, giving the US an opportunity to anticipate Japanese attacks and catch them off-guard.
That was what happened in the decisive naval battle of Midway, in which the US came out victorious, effectively putting an end to Japan’s blitzkrieg in the Pacific.

The tide now began to turn in favour of the Americans. Having defeated the Japanese in decisive battles both at sea and on land, and growing in strength by the day (thanks, in large part, to the American war effort), the US now focused on ceasing Japan’s occupied territories in order to cut their important supply lines of rubber and oil.
Around mid-1944, the US military successful launched a series of amphibious attacks on key strategic islands, such as the Solomon and Mariana islands. But these two important victories for the Allies came at a cost. More than ten-thousand American troops, many more Japanese troops, and thousands of civilians lost their lives.
The US then turned to the Philippines where they faced the terrifying Kamikazes (Japanese suicide units; see video above). After several months of fierce fighting, the US managed to retake the entire of the Philippines, but not without the loss of hundreds of thousands of combined American and Japanese troops and civilians.
In the early months of 1945, Japan was in a dire situation. Having been defeated at both sea and air, and suffering major territorial losses, the main Japanese islands were now dangerously cut off from vital supplies.
The Japanese, however, refused to admit defeat. Any attack to the Japanese islands was met with extreme brutality. In fact, Japan was convinced that if they continued fighting even more aggressively, the Americans would eventually concede, not being able to stomach it.
The US faced a major problem: how could they possibly defeat Japan once and for all without the continuing loss of American lives?

Initially, the US military employed strategic bombing raids, targeting Japan’s infrastructure and industrial areas. Despite some successes, accurate bombing was often thwarted by poor visibility and wind conditions. The American planes were also flying without a fighter escort and so were often easy targets for Kamikaze pilots.
The US then decided to employ a new bombing technique: incendiary bombing. Tokyo, Nagoya, Osaka and Kobe were among the targets of this firebombing, destroying much of the industrial facilities, and killed hundreds of thousands of Japanese citizens with many more made homeless.
In February 1945, the US invaded the island of Iwo Jima, which saw the deaths of about 7000 Americans and 18.000 Japanese. The Okinawa campaign a few months later was even bloodier, with a death toll of almost 100.000 Japanese soldiers, 15.000 American soldiers and about 40.000 civilians.
Even in the face of this calamity, the Japanese did not show any signs of wanting to surrender. The US pondered a full-scale invasion of the main island, but were concerned about the amount of casualties of such an invasion. The main Japanese island was defended by about one million men, supported by a few thousand airplanes. New Kamikaze pilots and even civilian volunteers were trained everyday to perform suicidal attacks on the potential invaders. With this intel, the US estimated that more than 200.000 American lives would likely be lost…
The US administration urgently needed a solution, and it would be a scientific breakthrough which would give them the trump card.

News of a classified scientific research project codenamed Manhattan Project reached the ears of US President Harry S. Truman.
While Truman was meeting Churchill and Stalin in Potsdam, Germany, he was informed of the success of Operation Trinity involving nuclear bombs, and ordered the manufacturing and dropping of the bombs on Japan immediately.
Two bombs were manufactured for that purpose: Little Boy and Fatman.
To understand the power unleashed by these bombs, let’s briefly go over some (very) fundamental chemistry and physics.
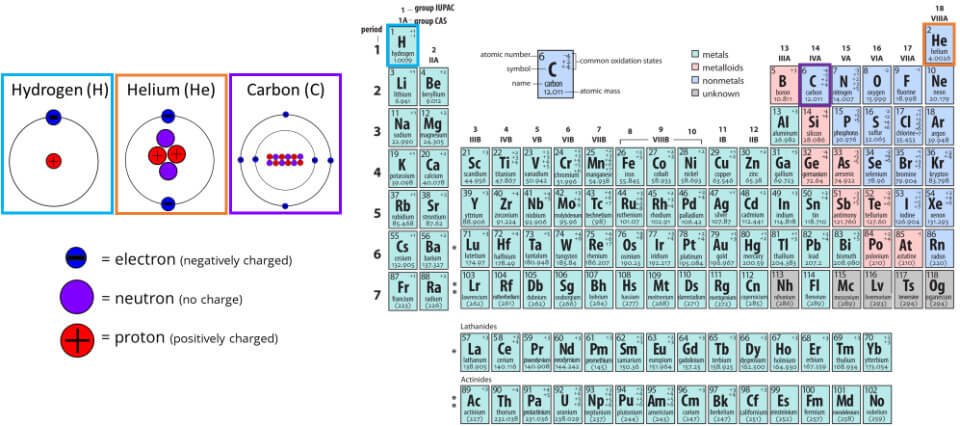
The way we tell different elements apart is by the number of protons in their atomic nucleus (i.e., their atomic number). So, hydrogen has 1 proton, helium 2 protons, carbon 6 protons, uranium 92 protons. You can see the atomic number of each element at the top left in the periodic table in the figure above.
Protons are positively charged and you might remember from your high-school physics class the old good force of attraction: like charges repel, unlike charges attract. The protons are tightly packed inside the nucleus of the atoms, so what prevents the protons from ejecting the nucleus?
The neutrons!
Just as protons, neutrons are also subatomic particles which, as the name implies, are electrically neutral. They can counteract the repulsive force of the protons and, thus, stabilise the nucleus. So, helium has 2 protons as well as 2 neutrons, carbon has 6 protons and 6 neutrons, etc.
As the number of protons starts to increase however, the repulsion between the protons will increase drastically. Take uranium for example. It has 92 protons, resulting in a massive repulsion. Adding only 92 neutrons would not keep these protons packed together; the nucleus would destabilise quickly and decompose into different, smaller and more stable atoms.
This process of disintegration from a bigger atom into smaller ones is called fission (the word “fission” comes from the Latin word fissiō, which means to break up or to divide), and the elements that result from fission are called the fission products.
So, the only way to make the nucleus of an uranium atom (U) stable is by adding a few more neutrons. Atoms with the same number of protons but with different amount of neutrons are called isotopes. Naturally occurring isotopes of uranium are U-238 (146 neutrons), U-235 (143 neutrons) and U-234 (142 neutrons).
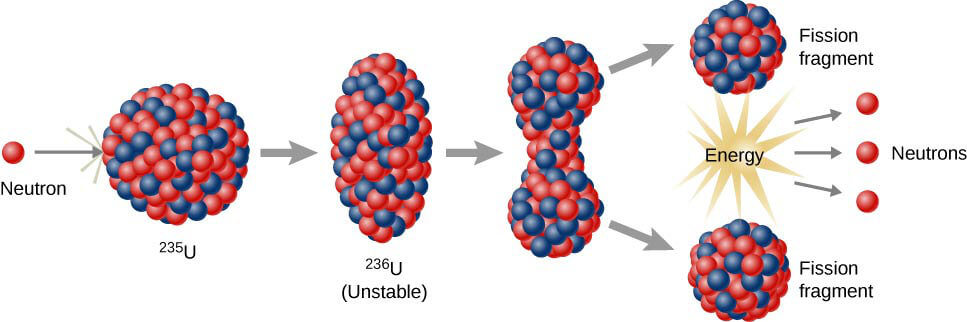
Shortly before WWII, scientists had discovered that it would be possible to force U-235 to fission by shooting a neutron into the nucleus.
They noticed that if a neutron is bombarded very quickly into the nucleus of a U-235 a series of processes happen:
1) The nucleus absorbs the neutron and turns into U-236 (because it gained one additional neutron it’s atomic mass is now 236).
2) The addition of a neutron completely destabilises the nucleus of the atom
3) This destabilization causes the atom to split into two smaller and more stable isotopic elements, e.g., barium-139 and krypton-94.
4) In the example above, Barium-144 has 88 neutrons, whereas krypton-94 has 54 neutrons. The U-236 had 144 neutrons, but 88 + 54 = 142. This means that in addition to those smaller elements, there are two (or sometimes three, depending on the elements produced) additional neutrons released.
5) The free-floating neutrons have the require speed to collide with other U-235 atoms and cause new fissions. This generates what is called a chain-reaction.
One of the biggest hurdles the Manhattan Project scientists faced was the presence of meagre amounts of U-235 (about 0.7%) in natural uranium.
And low amounts of U-235 meant that the free neutrons would be absorbed at a faster rate than they were created, and the chain-reaction would not be self-sustaining.
So, they had to enrich uranium sufficiently until it reaches a supercritical mass, that is, the amount of U-235 required to trigger a chain-reaction.
And it is this chain-reaction of U-235 what makes a bomb go… BOOM!!!
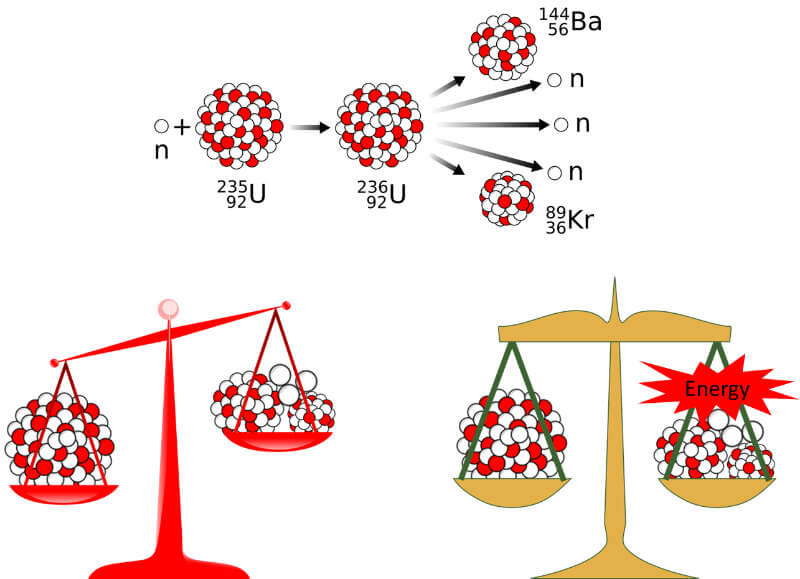
Now, this is important. Before fission, the atomic mass of the original U-236 is actually greater than the sum of the atomic masses of the fission products (see figure above). So where did the remaining mass go?
The answer: it has been converted into energy!
Remember, the only reason those protons and neurons are packed inside the nucleus is because there is a very strong force acting on them, the aptly named “strong force”. Thus, in order to break up a nucleus into smaller constituents you need energy – the binding energy. And larger nucleus have a higher binding energy. Once you are able to break the nucleus, this energy is also released.
So, in the example above, when fission splits the U-236 into the elements of Ba-144 and Kr-90 plus 2 neutrons, it also releases around 200 MeV (or 200 million electron volts). This amounts to about 3.2 x 10-11 Joules.
It doesn’t sound impressive, does it? After all, 3.2 x 10-11 Joules are a measly 0.0000000000032 Joules.
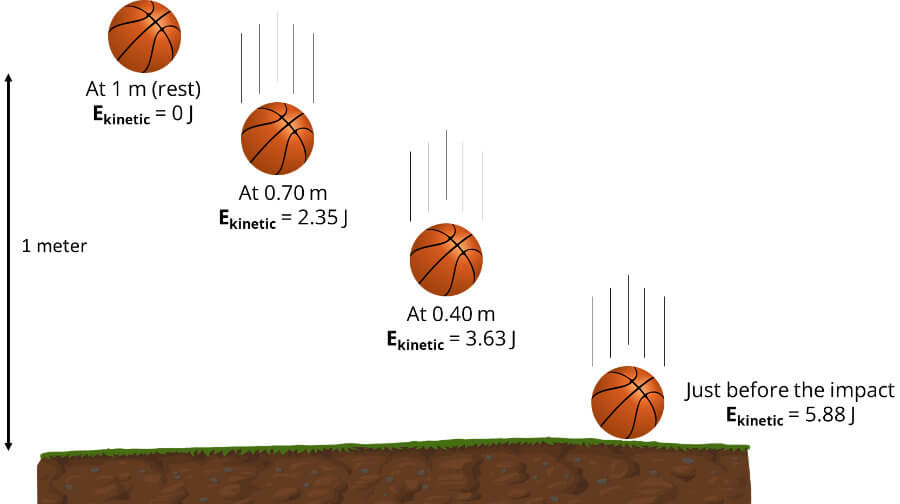
For comparison, if you drop a basketball from a height of 1 meter, the ball will have gained about 5.88 Joules just before it reaches the ground (see figure above) – that’s more than one hundred billion times larger than the energy released by the fission of a single atom of U-235. And if you explode one ton of TNT, it will release an astonishing 4184000000 (or 4.18 x 109) Joules worth of energy.
However, you need to realise that when the atomic bomb was dropped in Hiroshima, about 0.90 kg of uranium underwent fission in a matter of picoseconds. And 0.90 kg contains about 2.30 x 1024 of U-235 atoms! Simple math will tell us that if 2.56 x 1024 atoms of U-235 do fission, it will lead to the release of 4.61×1026 MeV or about 7.4 x 1013 Joules!
And that’s a massive amount of energy!!!
And, you see, there was about 64 kg of uranium in Little Boy, however only less than 2% actually became fissile! The rest was dud uranium.
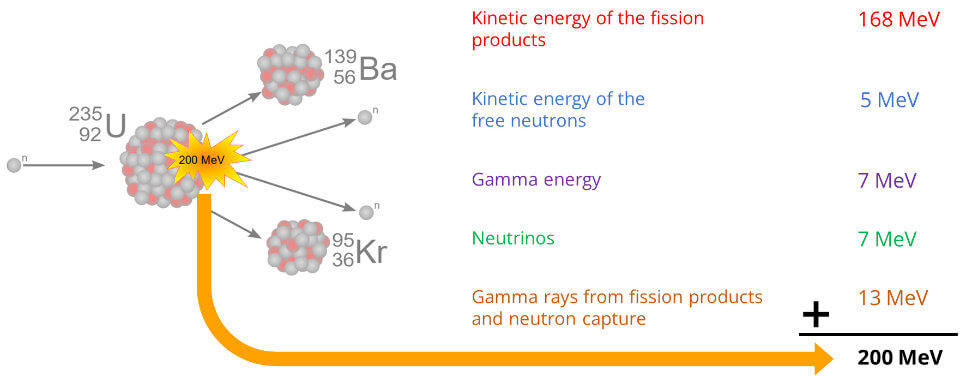
As explained above, when an atom of U-235 undergoes fission, it releases about 200 MeV of energy.
Out of these 200 MeV, the most part – about 169 MeV – is kinetic energy of the fission products. Other fission fragments that contain kinetic energy include free neutrons (about 5 MeV), gamma ray photons (about 7 MeV) and neutrinos (about 7 MeV).
What exactly comprises the 169 MeV of energy? Due to their nuclear charges, the two fission products will experience a very large repulsion and will tend to move away from each other. Thus, they will have kinetic energy.
Kinetic energy is the energy of motion. To put simply, if an object is moving, it will have kinetic energy (think of the basketball example I mentioned above), and the faster it moves, the higher it’s kinetic energy will be. A basketball will hurt your face more if it hits you at a faster speed, since there will be more kinetic energy that can be transferred to your face.
About 7 MeV are energy from immediate gamma rays. These are extremely energetic photons (light particles), and there will be so many released from a nuclear explosion that a very bright light will be seen even before any sound is heard (see below).
The remaining energy comes from gamma rays from fission products and neutron capture (total of about 13 MeV).
If you add all these up, you will come to approximately 200 MeV – the total energy released by the fission of a single U-235 atom (see figure above).
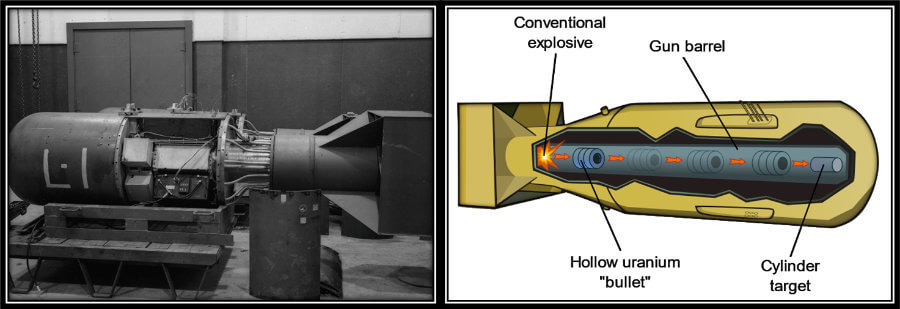
Little Boy (see figure above) consisted of a gun that fired a chunk of U-235 against another chunk of U-235, which resulted in a supercritical mass. Once the two masses of U-235 were joint, an initiator shot a series of neutrons, initiating the chain-reaction. The energy released was so immense that the bomb simply could not hold it in and blew itself apart.

Little Boy was transported to the Mariana Islands. There, in the morning of August 6th, Colonel Paul Tibbots flew his B-29 plane, Enola Gay, en route to Hiroshima carrying Little Boy.
At 8.15 a.m. Little Boy was released.
The amount of destruction caused is simply mind-blowing. Just for comparison, the blast of a single ton of TNT is sufficient to turn an entire building into rubble; anyone standing within a 20-meter radius of the center of the explosion will be killed immediately.
Little Boy was 15.000 times more powerful!
That was no typo! Little Boy had the power of 15.000 tons of TNT!
About 50% of the energy from the Little Boy came in the form of blast energy, 35% from thermal energy and the remaining 15% from nuclear radiation.
Blast effects were what caused much of the destruction in Hiroshima. The shock waves from the blast produced sudden and extreme changes in air pressure and very high winds. These waves travelled faster than the speed of sound, decimating anything within an 1.6-km (1-mile) radius. Anyone standing in their way would have their internal organs bursting instantly.
Beyond that distance damage was caused by collisions with flying bits of buildings and other debris.

Thermal energy is the type of energy emitted in the form of light and heat.
Even though the bomb detonated 600 m above ground level, the ground temperature just under the mushroom cloud (the hypocenter) was incredibly high, about 5000 degrees Celsius or 9032 degrees Fahrenheit, which is way more than the melting point of iron! Thousands of people were instantly vapourised.
Because thermal radiation travels at about the speed of light, people would have seen a very bright light even before they had the chance to hear any explosion. Anyone looking in the direction of the explosion would be susceptible to flash-blindness.
People also experienced skin burns by the extreme heat, the severity of which depended on how close they were to the hypocenter.

Nuclear radiation is the invisible, but just as deadly, form of energy from a nuclear explosion.
Most people in the critical blast area would have received lethal dosages of radiation. However, residual radiation would have also existed from the radioactive fallout, such as that produced from the fission products. Although some of these products only remain radioactive for a few seconds, others can be a hazard for many years.
In fact, in the weeks that followed the bombing, thousands of people died from radiation poisoning (e.g., from strontium-90). Sources regarding the death toll vary, but about 40.000 to 100.000 thousand people are estimated to have lost their lives.

Staggeringly, the Hiroshima bombing did not force Japan to surrender.
On August the 9th, only three days after Hiroshima, the second bomb, Fatman, was dropped on the military port of Nagasaki. If you thought Little Boy was powerful, Fatman was even more, the equivalent of 20 tones of TNT. About 35.000-50.000 people died from the explosion, not killing more and doing more damage only because it exploded way off target – somewhere over the Urakami valley, instead of over the city itself.
At about the same time of the Nagasaki bombing, the Soviet Union declared war on Japan and invaded Japanese-occupied Manchuria and northern China.
Japan’s position in the war had become untenable, so, on the 2nd of September 1945, Japan surrendered unconditionally.
Leave a comment
Add Your Recommendations
Popular Tags